More than 2 million people in the United States rely on Suboxone as part of their journey to overcome opioid addiction. While the medication has been praised for its effectiveness in managing withdrawal symptoms, troubling reports of severe dental damage and other side effects have surfaced, leaving many patients grappling with unexpected health complications. As a result, many injured patients are filing a Suboxone lawsuit to recover their financial losses.
If you or a loved one has experienced harm from Suboxone, you may be entitled to pursue legal action against its manufacturer. Lawsuits against Indivior, the pharmaceutical company behind Suboxone, allege that the risks of the drug were downplayed and patients were not adequately warned about its potential dangers.
Whether you've suffered unexpected dental decay, liver damage, or other complications, there is legal recourse available. At LitigationConnect, we are here to fight for the justice you deserve.
Don't wait to take the first step—connect with an experienced Suboxone lawsuit attorney today at (833) 552-7274 or complete our brief online form for a free consultation to explore your legal options.
Contact Our Team Today
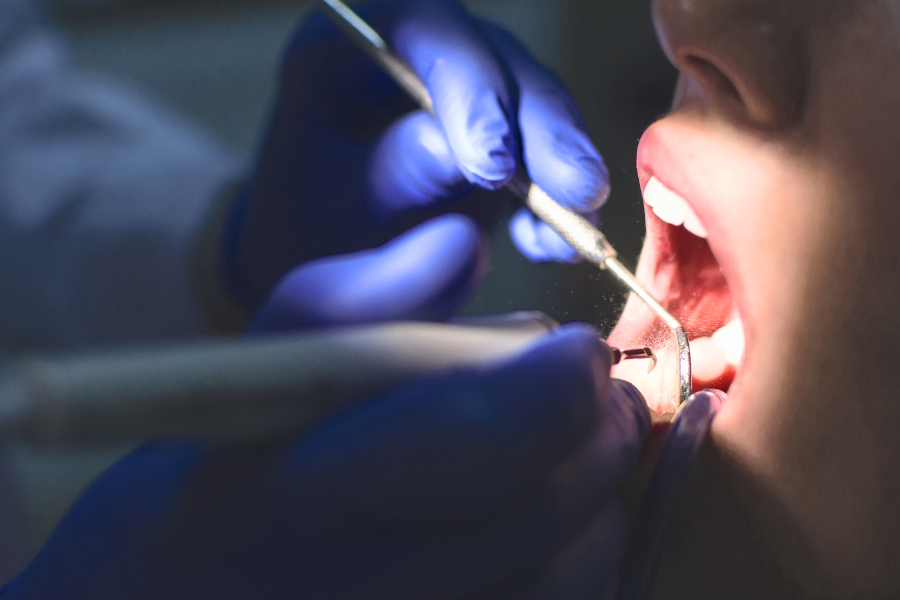
Don’t wait to take the first step—contact us today at (833) 552-7274 for a free consultation to explore your options.
Suboxone Lawsuit Updates - What is happening in the Suboxone Lawsuit?
December 1, 2025 - Suboxone MDL Lawsuit Count Slightly Drops
- As of early November 2025, there are 1,871 active Suboxone tooth damage cases pending in federal court.
- That’s a slight decline from October, due to dismissals related to missing documentation.
- New cases are still being filed, keeping the overall litigation active.
Judge Allows One Plaintiff to Proceed
- Plaintiff Michelle Robinson was allowed to continue her case despite missing earlier deadlines.
- She was incarcerated for over 8 months in 2025 and unable to complete litigation forms on time.
- After her release, she submitted the required documentation, and the court found good cause for the delay.
What This Means for Plaintiffs
- Courts are willing to consider legitimate hardships when delays are explained and corrected.
- Staying responsive and submitting forms remains crucial to maintaining active claims.
- The litigation is still moving forward with thousands of plaintiffs pursuing justice for Suboxone-related dental injuries.
- If you are considering your legal options after being harmed by Suboxone, contact Litigation Connect to learn more.
November 1, 2025 - New Court Orders Aim to Move Suboxone Cases Forward
- A federal judge overseeing the Suboxone MDL has issued new case management orders to improve efficiency.
- All new Suboxone lawsuits filed after October 1, 2025, must include a completed census form within 60 days.
- Older lawsuits must submit their forms by June 1, 2026.
Internal Company Documents Ordered for Production
- Indivior and Aquestive must turn over extensive internal records, including FDA filings, marketing materials, and reports on nearly 90 dental injury cases.
- The goal is to give plaintiffs access to critical evidence about what the companies knew.
More Than 11,000 Lawsuits Filed Nationwide
- An estimated 11,000 Suboxone users are claiming severe dental damage, including decay, erosion, and loss, because they were not adequately warned about these risks when prescribed the dissolvable Suboxone film.
- The FDA updated the warning label in 2022, but only after many people had already suffered harm.
Now Is the Time to Act
- If you’ve experienced dental damage from Suboxone, Litigation Connect can help match you with a lawyer who’s actively involved in this national litigation.
- Free consultations are available to help you understand your legal rights and next steps.
October 1, 2025 - Thousands of Injured Patients Join the MDL, Judge’s Patience is Wearing Thin
Suboxone Lawsuit Count Is Rising
- 1,882 cases are currently filed in the Suboxone MDL.
- Due to batch filings, the actual number of plaintiffs likely exceeds 20,000.
- Each batch filing can include up to 100 injured individuals.
Judge Cracks Down on Delays
- During a September 9 hearing, the judge warned both plaintiffs and third parties that delays won’t be tolerated.
- Three providers must now turn over medical records or risk Rule 45 contempt sanctions.
- Plaintiffs who missed key deadlines had their claims dismissed with prejudice, which means they cannot refile.
Plaintiff Census Moving to Round 2
- The court will soon issue a new order starting Census Round 2.
- This process helps identify which claims are fully supported and eligible for settlement or trial.
Suboxone Settlement Outlook
- Despite speculation, no settlement has been reached, and discussions have not really started.
- Realistic expectations now point to 2026, after early trial pressure builds on the defendant, Indivior.
What This Means for You
- If you're pursuing a Suboxone claim, it’s crucial to submit all paperwork and records on time.
- Without proof of prescription and injury, your claim could be dismissed.
- Free legal consultations are available to help guide you through the process.
September 1, 2025 - Important Court Ruling Helps Plaintiffs Build Cases, Large Groups of Lawsuits Filed
Recent Court Action
- August 26, 2025: A federal judge held a hearing targeting 4 providers who ignored court orders to turn over pharmacy records.
- No representatives from those providers showed up.
- The court is now considering sanctions and legal penalties for noncompliance.
Why This Matters
- Medical and pharmacy records are critical to proving a Suboxone case.
- Without records, lawsuits get delayed and trials can’t move forward.
- Nearly 20 pharmacies have been ordered to appear in court for ignoring deadlines.
New Lawsuits Filed
- 54 new plaintiffs filed a group lawsuit in the MDL this month.
- All claim serious dental injuries (tooth decay, erosion, loss) after taking Suboxone film as prescribed.
- The drug’s warning label was only updated in June 2022, which was too late for many users.
What Comes Next
- The MDL court wants to keep trials on track.
- The first bellwether trials depend on resolving this pharmacy record logjam.
- A global settlement cannot move forward until these records are produced.
Are You Considering Legal Action?
- If you used Suboxone and now suffer from dental damage, you may qualify for a lawsuit.
- Litigation Connect can match you with a knowledgeable Suboxone attorney for a free consultation.
August 1, 2025 - Recent Status Conference Recap
- A 2-hour Zoom hearing was held to review the Suboxone MDL progress.
- Parties reported several agreements:
- Handling of missing claim documentation
- Redaction process for past deposition transcripts
- Authorization forms for medical records
- Procedures for tracking post–October 7, 2024, claims
- Planning for additional document production
- Two disputes over Rule 30(b)(6) deposition language were resolved.
- One remaining production issue is still under negotiation.
Upcoming deadlines for the parties:
- August 15, 2025 – Plan for collecting non-custodial documents or identifying disagreements.
- September 29, 2025 – Submit fact sheets outlining claims and defenses.
- Next in-person hearing: September 9, 2025.
Deposition Protocol Finalized
- Applies to all fact witnesses and bellwether-related depositions.
- In-person format is standard; remote/Zoom allowed by agreement.
- Remote sessions require:
- Pre-arranged setup by requesting party
- Security and confidentiality measures
- Exhibit sharing before deposition when possible
- Deposing company employees: Defense must offer two dates within 10 days of plaintiff’s request.
- Only one lead attorney per side in most depositions.
- Plaintiffs may split questioning if notice is given.
Settlement Dynamics: Indivior’s Risk Exposure
- Indivior appears to be betting against jury sympathy for Suboxone users.
- Assumes stigma around addiction recovery will weaken plaintiff credibility.
- Risk: jurors may see dedicated patients harmed without proper warning.
- Many plaintiffs face:
- Full-mouth extractions
- Dentures in their 30s or 40s
- Extensive dental reconstruction expenses
- Failure to settle may result in large jury verdicts.
Mass Tort Momentum Building
- Suboxone MDL continues to grow and gain visibility.
- Plaintiff profiles: everyday people who are recovery patients with no prior dental issues.
- Damage stories are medically and emotionally compelling.
- A high-impact trial could significantly shift public and legal pressure.
Contact Litigation Connect for more information about the ongoing Suboxone litigation.
July 1, 2025 - Records Compliance, Plaintiff Replacements, and Settlement Timing
The federal Suboxone tooth decay litigation continues to evolve with new court activity affecting case progression and trial preparation. Below are the most recent developments as of early July 2025.
Pharmacy Record Compliance Under Scrutiny
Obtaining pharmacy records remains a significant obstacle for many Suboxone plaintiffs. Judge Calabrese issued a show-cause order against several companies for failure to provide records as requested. These companies include:
- Walgreens
- Safeway
- Porch Light Health | Front Range Clinic
These providers were accused of failing to comply with Case Management Order No. 13, which mandates that pharmacy records be produced within 30 days of receiving valid authorizations.
Update:
- The judge withdrew the order for Safeway and Porch Light after confirming they had complied.
- The hearing remains pending for Walgreens, where further enforcement action may be considered.
Plaintiff Substitutions in the Bellwether Trial Pipeline
On June 5, the court issued Second Amended Case Management Order No. 16, replacing 48 plaintiffs in the 500-member Records Collection Pool. Reasons for removal included:
- Voluntary dismissals
- Failure to submit required documentation
Replacements were randomly selected in accordance with pre-established protocol. Maintaining this pool ensures the MDL stays on schedule for case progression and potential bellwether trials.
Suboxone Settlement Expectations Remain Grounded
There’s ongoing speculation about a possible early settlement, even before upcoming Daubert (expert witness) rulings. However, based on the current strategy and defense behavior, an early resolution remains unlikely with the statute of limitations filing deadline quickly approaching.
The defendants are holding off because early settlement could have triggered an influx of new claims—waiting until after the deadline helps limit exposure.
Late 2025 is a more realistic estimate for the start of meaningful Suboxone settlement negotiations.
June 1, 2025 - Suboxone Lawsuits Move Closer to Trial
Nearly 900 people have now filed lawsuits claiming that Suboxone film, a medication used for opioid recovery, caused serious tooth decay and dental problems. These injured plaintiffs are asking the court to hold Suboxone's manufacturer, Indivior, responsible because it never warned potential users about the risks.
New Trial Process Announced
A new court order has laid out the path to the first trials. Here's the plan:
- 500 cases will be reviewed for medical records.
- 100 cases will move into deeper discovery.
- From there, 15 will be picked for trial consideration.
- Eventually, 4 individual cases will be selected to go to trial first.
These early cases—called bellwether trials—will help show how juries respond and could influence settlement talks.
The Plaintiffs are Real People, Suffering Real Harm
Many plaintiffs facing recovery took Suboxone to stay clean. However, they claim the drug's side effects led to crumbling teeth, chronic pain, and costly dental bills. Lawyers warn that if Indivior, the drugmaker, underestimates the compassion of jurors, it could cost them much more than a fair settlement now.
More Lawsuits Expected
With new rules allowing 100 plaintiffs per filing, expect a big wave of new cases in June. If Suboxone caused dental harm to you or someone you love, reach out to our team to learn more about your legal rights.
May 1, 2025—Suboxone Tooth Injury Lawsuits Advance in Federal MDL
Hundreds of lawsuits claiming that Suboxone causes serious dental injuries—including tooth decay and tooth loss—are now consolidated in a federal multidistrict litigation (MDL) proceeding. As of May 1, 2025, approximately 896 cases are pending in MDL No. 3092, overseen by Judge J. Philip Calabrese in the Northern District of Ohio. While no settlements or trials have occurred, pretrial work is steadily progressing.
Court Actions and Current Status
To manage the growing number of filings, the court approved Case Management Order No. 14, which allows up to 100 plaintiffs to file jointly under a single complaint. This procedural change is designed to reduce delays and streamline the administration of the MDL.
By mid-April, attorneys had identified a group of 500 plaintiffs for targeted discovery. This includes gathering dental treatment records and other documentation that could help determine which claims will be selected for early test trials. The court held a status conference on April 17, 2025, to address pending issues and maintain momentum.
Discovery Efforts and Upcoming Depositions
Plaintiffs' counsel have signaled plans to conduct corporate depositions under Rule 30(b)(6). These depositions will likely focus on how Indivior, the manufacturer of Suboxone, responded to dental complaints, what led to the FDA-mandated label change in 2022, and how the company’s internal structure relates to the design and marketing of Suboxone.
The court is also working to ensure that third parties cooperate with the discovery process. During the April hearing, the judge scheduled a show-cause hearing for a national pharmacy chain that had failed to provide relevant prescription records—records that may be essential to proving patterns of use and injury.
Although trial dates have not been scheduled yet, the litigation is entering a more advanced phase. Discovery is active, and groundwork is being laid for potential bellwether trials later in 2025.
March 20, 2025 - Filing Fees Might Drop for People Bringing Suboxone Lawsuits
Judge Calabrese just made things a bit easier for people looking to file Suboxone-related Big news for folks thinking about filing a Suboxone lawsuit: Judge Calabrese just signed off on an order that could make the process more affordable. Normally, each federal case costs $405 to file. But now, up to 100 plaintiffs can be included in a single complaint, with just one filing fee. That means major savings for individuals who were otherwise priced out of pursuing justice.
On top of that, the judge is allowing electronic bulk-filing of cases, which will make it easier for attorneys to organize everything and track what kinds of injuries are being claimed. With tens of thousands of potential cases still out there, this update could open the door for many more people to take action.
March 18, 2025 - New Judge's Order Highlights Just How Big the Suboxone MDL Has Become
Judge Calabrese recently signed an order that's going to make life a lot easier for attorneys handling Suboxone tooth decay lawsuits. Now, up to 100 plaintiffs can file their claims together in a single complaint, paying just one filing fee. This might sound like a small detail, but it speaks volumes about how massive this litigation really is.
We’re expecting yet another wave of new lawsuits this summer as the three-year statute of limitations deadline hits in several states. Right now, the drug makers behind Suboxone are already dealing with over 10,000 lawsuits alleging they failed to warn people about serious dental damage linked to their film strips. That's thousands of real people who've suffered broken teeth, tooth loss, and extensive dental work—and more claims are on the way.
Meanwhile, preparations for the first Suboxone bellwether trials are already underway. These early trials are a big deal because they'll give everyone involved a sense of how juries might react and what type of compensation jurors think plaintiffs deserve. Ultimately, the bellwether outcomes will play a huge role in deciding how thousands of other claims get resolved.
March 14, 2025 - Suboxone Lawsuit Takes a Step Toward Test Trials
Progress is picking up in the Suboxone litigation. Judge Calabrese has laid out the process for selecting bellwether cases—the handful of “test” trials that will help shape how the rest of the lawsuits are handled. The first step? Randomly choosing 500 cases and reviewing their records. From there, the court will go through a few rounds of narrowing down until a lead case is chosen to go to trial.
Bellwether trials are important because they give both sides a preview of how juries might respond to the evidence. It’s a key step that often leads to serious settlement talks down the line.
March 13, 2025 – Judge Orders Faster Production of Medical and Prescription Records
Judge Calabrese has issued a new order to expedite the production of medical and prescription records in the Suboxone MDL. The ruling requires pharmacies and medical providers to comply with record requests within 30 days.
It also mandates the acceptance of ink or electronic signatures for authorization and prohibits the demand for additional documents, such as ID copies, before releasing records.
This decision addresses significant delays that have hindered case progress and made it difficult to select bellwether trial candidates. By removing these obstacles, the court is ensuring plaintiffs have a fair chance to build their cases efficiently and keep the litigation moving forward in the Suboxone tooth decay MDL.
March 3, 2025 - Judge Steps In to Help Suboxone Plaintiffs Get Dental Records
One of the major hurdles in filing a Suboxone lawsuit—especially for people with dental injuries—has been getting access to dental records. Some dental offices have been dragging their feet or flat-out ignoring requests from patients and their attorneys. To move things along, Judge Calabrese issued an order clarifying what kind of authorization is needed for records to be released. He also made it clear that dental providers who don’t comply could face legal consequences, including contempt of court.
These records are key evidence in tooth damage claims, and without them, it’s nearly impossible to move a case forward. This order is a strong push to make sure survivors aren’t being stalled by paperwork issues.
February 25, 2025 - Judge Suggests Extending Deadline for Filing Suboxone Cases
As the window to file Suboxone-related lawsuits starts to close, Judge Calabrese is encouraging Indivior—the company behind the drug—to agree to a tolling arrangement. What that means is pretty straightforward: it would pause the countdown on filing deadlines and give people more time to prepare their cases.
Without it, lawyers may need to rush and file every potential case, even the ones that aren’t fully ready, just to meet the cut-off. A tolling deal would give more time for solid cases to come together and avoid clogging up the system. It’s a move that could benefit both sides in the long run.
February 6, 2025 – Defendants Request Bifurcation of Trials
Defendants in the Suboxone case have requested that the court bifurcate (or separate) the liability and damages stages of the upcoming trials.
If this request is granted, it would mean that juries would first need to decide whether Suboxone manufacturers are legally responsible (aka whether Suboxone is actually the cause of the tooth decay [psst...science says it is]) and the juries can hear separate evidence about individual damages if the initial causation stage is successful.
This strategy is old and tiring. The defense wants to separate the jury from the emotional elements of people being injured, suffering, and having their lives destroyed, while also delaying the procedures and potentially causing some plaintiffs to settle for less because they will get sick of waiting.
Almost ALL defense attorneys try this.
The judge’s decision on this request will be important, as it could impact the flow and strategy of future trials.
February 1, 2025 – Court Orders Mediation Before First Bellwether Trial
In an effort to encourage resolution, the judge has ordered mediation between plaintiffs and defendants before the first bellwether trials begin. This move suggests the court wants both sides to explore settlement options before fully committing to trial.
Mediation could lead to early resolutions/settlements for certain plaintiffs, but given the size and complications of this litigation, a global settlement is still uncertain.
If mediation proves unsuccessful, the trials will proceed as scheduled, providing more insight into how juries might respond to the allegations against the manufacturers. Either way, this order introduces a potential off-ramp for some litigants before the trials begin.
January 25, 2025 – FDA Submits Additional Data on Dental Injuries
The FDA has contributed new evidence to the Suboxone MDL, supporting the claims that Suboxone can cause severe dental damage.
This data from the FDA includes additional reports of tooth decay, enamel erosion, and related oral health issues among patients prescribed the drug. The agency’s findings may strengthen the plaintiffs’ arguments, particularly when it comes to causation, and could influence the upcoming expert testimony. With this data as backup, plaintiffs might just find it easier to establish liability and increase settlement leverage.
Indivior is expected to challenge the FDA’s findings, possibly arguing alternative causes or questioning the methodology behind the data collection.
January 18, 2025 – New Requirements Issued for 'Plaintiff Fact Sheet'
The court has tightened its standards for the plaintiff fact sheet (PFS) in the Suboxone case. This change is likely meant to streamline discovery and ensure consistency across cases.
Plaintiffs will now need to provide more detailed medical histories, more thorough proof of Suboxone usage, and evidence linking their dental injuries to the medication.
These changes are going to cause some delays and will definitely end up lowering the number of overall cases.
What is a Plaintiff Fact Sheet (PFS)?
A Plaintiff Fact Sheet (PFS) is a standardized document used in MDL and mass tort cases to make the discovery process easy and uniform. Instead of traditional interrogations and requests for this and that, a PFS provides essential information about each plaintiff’s claims, and all the information is in the same order and answers the same questions.
The PFS helps both sides assess the merits of each case and determine whether to proceed with that case or not.
What's included in a Plaintiff Fact Sheet?
While the exact requirements vary depending on the case and the court's orders, a PFS typically includes:
- Personal Information – Name, contact details, date of birth, and Social Security number.
- Medical History – Health conditions, history of dental or other injuries, and relevant past treatments.
- Medication Usage Information – When and how the plaintiff used the medication (e.g., Suboxone), dosage, duration, and prescribing physician details.
- Injury Details – Specific allegations about how Suboxone or another product harmed the plaintiff, including symptoms, diagnoses, and impact on daily life.
- Economic Damages – Details and exact figures on the medical bills, lost wages, and other financial losses attributed to the injury.
- Alternative Causes – If the plaintiff has any pre-existing conditions or other risk factors that could have contributed to their injuries, these must also be listed.
January 10, 2025 – Official Selections For Which Cases Will Be Bellwether Trials Scheduled for Summer 2025
The court has set a deadline for selecting the bellwether trials, which will be a big step in the Suboxone MDL.
These early test cases will help to give both the defense attorneys and the plaintiff attorneys an understanding of how juries might respond to the evidence and overall vibe of the case. This information is invaluable.
Whatever the juries decide during the bellwether trials will shape either settlement discussions or trial strategies, depending on the outcome.
Ideal Plaintiff Example: If the juries show a tendency to believe the Suboxone tooth decay science and seem to sympathize with these people who were trying to become better members of society, things could look good for the plaintiffs. Once these bellwether juries come back with their final trial award decisions, and they are high, it will definitely push Indivior to settle rather than risk every other trial going the same way.
Ideal Defendant Example: If the bellwether juries don't seem to understand the laws and science involved in the Suboxone MDL—it's presented in a confusing way, and things don't make much sense—and they have more of an attitude that lacks empathy for people looking to recover from drugs than things could go well for the defense. This would likely give Indivior the edge when it comes to settlement negotiations, and they may decide to take the cases to trial anyway in the hope that they will get
Both plaintiffs and defendants will have a say in the selection process, with criteria mostly focusing on the severity of injuries, facts and patterns, and jurisdictional issues. Whatever the situation, this clearly shows signs that the Subxone MDL is moving into its more active stage, where things will move a little quicker.
January 8, 2025 - Judge Rules Key Claims Can Proceed in Suboxone MDL
On December 31, 2024, Judge Calabrese delivered a major win for plaintiffs in the Suboxone MDL. The Court denied the defendants’ motion to dismiss key claims, allowing 'pre-approval design defect' and 'post-label-change failure to warn' claims to move forward. The judge noted evidence that adverse event reporting continued even after the label update in June 2022.
While the Court dismissed 'post-approval design defect' claims, the ruling keeps the core of the plaintiffs’ case intact. Discovery will now focus on what the defendants knew about the risks of Suboxone film and when they knew it.
This is a big step toward holding Reckitt Benckiser and Indivior accountable for marketing Suboxone without adequate dental risk warnings. It’s another reminder that corporations cannot avoid responsibility for the harm their negligence causes.
October 1, 2024 - E-Discovery Guidelines Issued in Suboxone MDL
The Court issued Case Management Order No. 11, outlining protocols for electronic discovery in the Suboxone MDL. This ensures the preservation, organization, and sharing of key documents, including emails and spreadsheets, between the parties.
The order emphasizes collaboration to streamline the process and prevent unnecessary court interventions. It also provides guidelines for managing non-party documents and complex files like databases.
This move underscores the Court’s intent to keep the MDL efficient while balancing the discovery burdens on all parties.
September 12, 2024 - Some Indivior and Reckitt Benckiser Subsidiaries Released from MDL
Judge Calabrese has dismissed several subsidiaries from the Suboxone MDL, including Indivior PLC, Reckitt Benckiser LLC, and Reckitt Benckiser Healthcare (UK) Ltd. However, key defendants like Aquestive Therapeutics and the main Indivior entities remain involved.
This decision narrows the case but ensures the focus remains on the companies most responsible for Suboxone’s design, marketing, and labeling.
August 23, 2024 - Defendant Indivior Files Motion To Dismiss
Defendants filed a partial motion to dismiss against plaintiff Ryan Bennett, claiming the doctrine of federal preemption completely preempts his design defect claims. Indivior also claims parts of the plaintiff's failure to warn claim are also preempted. The doctrine of federal preemption states that when state law interferes or conflicts with federal law, federal law will supersede the state law.
Motion to Dismiss Based on Failure to Warn Claims
Indivior contends that Ryan Bennett's failure to warn claims are preempted because the label was approved by the FDA, aka the U.S. Food and Drug Administration—clearly a federal body. Remember, the federal law overrules the state law concept? Thus, they allege that an approved label is legally adequate.
Unfortunately for Indivior, their argument fails based on legal precedent, Wyeth v. Levine, which says that manufacturers remain responsible for their labels at all times. This means Indivior, or any manufacturer, remains responsible for updating their own label at all times, regardless of whether it was once approved by the FDA in the past.
Motion to Dismiss Based on Design Defect
Indivior also filed a motion to dismiss for the design defect claim. Again, the reason is complicated, but basically, it comes down to the idea that Indivior believes the defective design of the Suboxone film strips is theoretical since, to prove the defect, one would need to guess whether the FDA would approve a safer alternative.
Fortunately, we do not need to speculate what the FDA may or may not do. The FDA has already approved a safer alternative to Suboxone film strips: Sublocade, an injectable version of buprenorphine, the same medicine in the film strips.
Pharmaceutical Companies Always Try This
Do you know what is so frustrating about these cases? These giant pharmaceutical companies spend hundreds of millions on these beautiful advertisements you and I see everywhere, claiming how great their medicine is and how it will change your life. But if they are negligent, put profits over safety, or fail to warn consumers about serious side effects, they jump right to their playbook and do EVERYTHING they can to avoid being held responsible for their mistakes.
They are literally following this format:
- Find an illness that hurts people.
- Convince those people that you have the perfect solution.
- Sell them the solution at an astronomical price.
- If the solution ends up causing more harm, they fight like hell to say that steps 1-3 were not their fault.
These drug manufacturers always file a motion to dismiss and use the federal preemption strategy in every single lawsuit against them. And this is just one page of their giant playbook on how to get out of being held accountable for their harmful actions.
It’s really quite infuriating.
August 18, 2024 - Research Highlights Suboxone’s Dental Risks
A recent study in The Journal of the American Medical Association underscores the serious dental harm caused by sublingual Suboxone compared to other buprenorphine formulations. Users experienced significantly higher rates of tooth decay and dental injuries.
This evidence bolsters plaintiffs' claims that Indivior’s label failed to adequately warn patients and doctors about Suboxone’s dental risks. It will be a critical focus as the MDL moves forward.
August 1, 2024 - Indivior’s Motion for Partial Dismissal in Suboxone MDL
Indivior has filed a motion for partial dismissal, relying heavily on federal preemption arguments. They claim that FDA approval of Suboxone’s design and labeling protects them from liability.
Their primary arguments include:
- Design Defect Preemption: They argue federal law overrides state claims because any design change would need FDA approval.
- Failure to Warn: They assert that the FDA’s initial label approval and the lack of "newly acquired information" post-2022 protect them.
- Non-NDA Holder Claims: They claim only the New Drug Application (NDA) holder can be held responsible, shielding certain entities.
Indivior’s motion seeks to dismiss these claims with prejudice, aiming to avoid future challenges. However, plaintiffs are confident these arguments won’t hold up under scrutiny.
July 20, 2024 - Judge Orders Indivior to Produce Antitrust Documents
Judge Calabrese has directed Indivior to turn over key documents from its antitrust litigation, which concluded in October 2023. These records are expected to shed light on the company’s marketing tactics and decision-making processes.
Indivior previously paid $385 million to settle antitrust claims, where it was accused of pulling Suboxone tablets from the market to avoid generic competition. Additional documents from patent litigation, which resulted in a $102.5 million settlement, will also be produced.
This order will help plaintiffs demonstrate how Indivior prioritized profits over patient safety by rushing Suboxone film to market without adequate testing of its dental risks. The next status conference is set for September 4, 2024, and we anticipate further developments then.
June 26, 2024 - Judge Rejects Indivior’s Push for Phased Discovery
In a significant win for plaintiffs, Judge Calabrese has denied Indivior’s motion to bifurcate discovery. Indivior had sought to separate general and specific causation, a move that would have delayed the litigation by up to 18 months.
The judge recognized that general and specific causation are intertwined and should proceed concurrently. This decision keeps the MDL on track and demonstrates the Court’s commitment to judicial efficiency. With this ruling, the Suboxone litigation is poised to gain momentum.
June 14, 2024 – What is the Statute of Limitations in the Suboxone MDL?
Because the statute of limitations (deadline to file a claim) varies from state to state, there has been much confusion over two things:
- Which states have an expired statute of limitations (SOL) and which are still within the deadline?
- And how can the court prevent a situation in which thousands of attorneys file all their Suboxone claims before vetting them just to make sure they meet the deadline?
After much back and forth, here is a breakdown of the statute of limitations for the Suboxone MDL:
- FDA Warning and Label Update: In June 2022, Suboxone's warning label was updated to reflect the dental health risks. This set the clock on failure-to-warn claims for states with a two-year SOL, making their deadline June 2024.
- Tolling Agreements: To address the concerns about the upcoming SOL deadlines, the plaintiffs in the Suboxone MDL sought tolling agreements. A tolling agreement effectively pauses the SOL to allow more time for potential plaintiffs to file claims and vet existing claims. The defendants initially resisted, causing approximately 10,000 Suboxone lawsuits to be filed simultaneously on June 14, 2024.
- State-Specific SOL Variations: Moving forward, you need to know the SOL deadline for your state; they range from one to four years for these types of product liability claims. For instance, states with a two-year SOL had a filing deadline of June 2024, while those with a three-year SOL have until June 2025, and those with a four-year SOL have until June 2026.
- States with Three-Year SOL:
- Arkansas
- Maryland
- Massachusetts
- Michigan
- Montana
- New Hampshire
- New Mexico
- New York
- Rhode Island
- South Carolina
- South Dakota
- Vermont
- Washington
- Washington D.C.
- Wisconsin
- States with Four-Year SOL:
- Florida
- Nebraska
- Utah
- Wyoming
*It's important to note that the SOL can vary based on individual circumstances and specific state laws. So speak with a qualified mass tort attorney involved in the Suboxone MDL to ensure your case qualifies with applicable deadlines.
June 10, 2024 - Arguments Heard on General vs. Specific Causation
On June 6, 2024, Judge Calabrese heard oral arguments on whether to bifurcate general and specific causation issues in the Suboxone MDL. Indivior argued for staged discovery, claiming it would simplify the case.
Plaintiffs strongly opposed the motion, emphasizing that bifurcation would unnecessarily delay the process and burden plaintiffs with additional procedural hurdles. The LLN team believes this is a stalling tactic by Indivior to frustrate plaintiffs and discourage participation in the MDL.
General causation, or whether Suboxone causes dental injuries, is already supported by FDA findings and label changes in 2022. Plaintiffs argue this issue should not delay the simultaneous consideration of specific causation for individual claims.
May 20, 2024 - Judge Sets Deadline for MDL Plaintiff List Amid Tolling Dispute
With no agreement reached on tolling, Judge Calabrese has ordered the plaintiff steering committee to submit a master list of plaintiffs by June 14, 2024. This step ensures that all claims are preserved before the statute of limitations expires in states with a two-year deadline.
Defendants must respond by July 1, 2024, to determine if cases should remain in the MDL or proceed in state courts. While tolling agreements are typically used to streamline MDLs, Indivior’s refusal to cooperate has created additional challenges. Despite this, the Court’s intervention aims to prevent procedural delays and protect the rights of affected plaintiffs.
May 1, 2024 - Discovery Structure and Tolling Agreements Take Center Stage
During the latest status conference, Judge Calabrese heard arguments on discovery protocols and the potential for a tolling agreement. Indivior is pushing for staged discovery focused solely on general causation, which plaintiffs argue is unnecessary given the strong evidence linking Suboxone to dental harm.
Tolling agreements, which could extend filing deadlines and reduce unnecessary lawsuits, remain a contentious issue. Plaintiffs have offered to provide proof of Suboxone use through pharmacy or medical records, but Indivior has rejected this proposal.
The absence of a tolling agreement risks overwhelming the MDL with unvetted claims, creating inefficiencies for both sides. The next steps in discovery will be critical in determining how this litigation progresses.
April 26, 2024 - Indivior Attempts to Delay MDL with General Causation Focus
Indivior is asking the Court to prioritize general causation in the Suboxone MDL, a move many see as an attempt to stall the litigation. General causation addresses whether Suboxone causes dental injuries, an issue already supported by the FDA’s 2022 label change.
Plaintiffs argue that both general and specific causation should proceed together to avoid unnecessary delays. Indivior’s focus on general causation appears to be a tactic to wear down plaintiffs and limit the number of claims filed.
Despite these challenges, new Suboxone lawsuits continue to be added to the MDL, reflecting the growing awareness of the drug’s dental risks.
March 30, 2024 - Suboxone Case Criteria Updated to Focus on Severe Dental Injuries
The Lawsuit Legal News team has revised its case intake criteria for the Suboxone MDL. Moving forward, cases must involve three or more tooth extractions or significant tooth loss directly linked to sublingual Suboxone use.
This decision ensures that only strong cases are filed into the MDL, aligning with the goal of maintaining the integrity of the litigation. Plaintiffs with qualifying cases should act promptly to understand the specific statute of limitations in their state.
March 22, 2024 - Direct Filing Now Allowed in Suboxone MDL
Under Case Management Order No. 3, Judge Calabrese has permitted the direct filing of lawsuits into the Suboxone MDL. This move eliminates delays associated with transferring cases from other jurisdictions, improving efficiency for plaintiffs and the Court.
While plaintiffs’ leadership is cautious about filing cases with minimal injuries, the MDL is expected to grow rapidly as the statute of limitations deadline approaches for states with two-year filing windows. We anticipate a significant surge in claims over the next two months.
March 10, 2024 - Leadership Appointed in Suboxone MDL
The first status conference for the Suboxone MDL took place on March 7, 2024, before Judge Philip Calabrese. At this hearing, Stanley Gipe of Dolman Law Group was appointed to the Plaintiff’s Steering Committee, which will help lead the litigation.
With the FDA’s 2022 label update linking sublingual Suboxone to severe dental injuries, this litigation has strong scientific backing. We expect rapid growth in the MDL as more plaintiffs come forward and the statute of limitations nears for states with shorter deadlines.
February 17, 2024 - Initial Status Conference Scheduled for Suboxone MDL
Judge Calabrese has scheduled the first status conference in the Suboxone MDL for March 7, 2024, in Cleveland, Ohio. The agenda includes appointing leadership for both plaintiffs and defendants and establishing a discovery calendar.
This litigation is expected to move quickly due to the strong scientific evidence linking Suboxone to severe dental issues. Attorneys seeking leadership positions must attend in person and submit applications by March 1, 2024.
February 7, 2024 - Suboxone Lawsuit Gains Momentum
Advertising for Suboxone lawsuits is ramping up across social media platforms, reflecting growing awareness of the litigation. This mass tort is poised to become one of the largest of 2024, driven by the mounting evidence of Suboxone’s dental risks and the sheer number of affected individuals.
February 2, 2024 - Suboxone Lawsuits Consolidated into MDL
The Judicial Panel on Multidistrict Litigation (JPML) has officially consolidated Suboxone lawsuits into a Multidistrict Litigation (MDL) before Judge Philip Calabrese in the Northern District of Ohio. This streamlines pretrial proceedings while allowing individual claims to retain their independence.
The agreement to consolidate reflects the strength of the cases and the growing number of plaintiffs alleging dental injuries caused by Suboxone film.
December 26, 2023 - JPML to Consider Suboxone MDL Consolidation
The JPML has scheduled a hearing for January 25, 2024, to decide on consolidating Suboxone lawsuits into an MDL. This would likely centralize the litigation in the Northern District of Ohio, a favorable venue for these cases.
Consolidation would streamline discovery and pretrial rulings, ensuring consistency across claims while reducing the burden on individual courts.
September 28, 2023 - First Suboxone Lawsuit Filed Over Dental Injuries
The first Suboxone lawsuit alleging dental injuries has been filed in the Northern District of Ohio by David Sorensen. The plaintiff claims that Suboxone’s sublingual film caused severe tooth decay, leading to significant medical expenses.
This case marks the beginning of what is expected to become a major mass tort, with plaintiffs accusing Indivior of rushing Suboxone film to market to avoid generic competition after its tablet patent expired.
June 1, 2022 - FDA Requires Suboxone Label Change for Dental Risks
The FDA has updated the warning label for sublingual Suboxone to include risks of severe dental injuries such as tooth decay, oral infections, and total tooth loss. This change followed a safety communication in January 2022 and reflects growing concerns about the drug’s impact on dental health.
This label update is a critical piece of evidence in the Suboxone litigation, as it directly links the drug to serious dental injuries.
January 12, 2022 - FDA Warns of Dental Risks Associated with Suboxone Film
The FDA issued a safety communication warning the public about severe dental injuries linked to sublingual Suboxone, including tooth decay, infections, and tooth loss. Despite acknowledging the risks, the FDA stated that Suboxone’s benefits for treating opioid addiction outweigh the harm.
Plaintiffs argue that these risks were not adequately communicated to patients or healthcare providers, setting the stage for future litigation against Indivior.
What is Suboxone?
Suboxone is a prescription medication approved by the FDA in 2002 for opioid addiction treatment. It is a combination of two active ingredients:
- Buprenorphine is a partial opioid agonist that interacts with brain receptors to reduce opioid cravings and withdrawal symptoms without the high associated with opioids like heroin.
- Naloxone is included to deter misuse. If injected, naloxone can induce withdrawal symptoms to prevent abuse.
Suboxone's sublingual film or tablet was designed to dissolve under the tongue, offering a convenient way to aid recovery. It became a crucial part of Medication-Assisted Treatment (MAT), a therapy that blends medication with counseling and behavioral support to help patients overcome opioid use disorder.
While Suboxone has successfully helped millions manage opioid dependency, troubling side effects and safety warnings have raised serious concerns that may lead to compensation for those injured by this product.
Why Choose Suboxone Mass Tort Lawyers From the LitigationConnect Network of Legal Professionals?
At LitigationConnect, we are dedicated to fighting for victims of dental injuries caused by Suboxone. When you partner with our network of Suboxone lawsuit attorneys, you benefit from:
- Experienced Legal Team: We partner with law firms boasting extensive experience in Suboxone MDL cases and mass tort litigation. They understand the legal strategies required to hold Indivior accountable for the harm its product has caused.
- Nationwide Representation: With Suboxone mass tort attorneys located nationwide, we can help you file a claim before your state's statute of limitations expires.
- Long History of Successful Victories. Our network of legal professionals has helped thousands of clients secure significant recoveries that help manage long-term care needs and facilitate an easier journey toward recovery.
With statutes of limitations varying by state and the complexities of mass tort litigation, time is of the essence. Don't face legal challenges alone. Take control of your future today by calling (833) 552-7274 or completing our online form for a free consultation with an experienced Suboxone lawsuit lawyer.
What Side Effects May Qualify for Compensation in a Suboxone Class Action Lawsuit?
Suboxone has helped many individuals in their fight against opioid addiction, but its use has been linked to significant side effects, many of which are central to ongoing litigation.
If you've experienced harm, you may be eligible to join a Suboxone lawsuit and seek compensation. Below are the key side effects being considered:
- Severe dental problems: Suboxone's sublingual design can lead to tooth decay, cavities, gum disease, and tooth loss. The prolonged exposure of its active ingredients to the mouth contributes to weakened tooth enamel and rapid dental deterioration, even with good oral hygiene. These injuries were highlighted in the FDA's 2022 warning and are now a leading cause of Suboxone tooth decay claims.
- Respiratory depression: Suboxone's opioid properties can slow or suppress breathing, particularly when used alongside sedatives or in individuals with pre-existing respiratory issues. This side effect may pose life-threatening risks if left unaddressed.
- Liver damage: Users have reported liver inflammation and, in severe cases, liver failure. Those with prior liver conditions or who consume alcohol are especially vulnerable to this risk while on Suboxone.
- Dependency or misuse: Despite its purpose as an addiction treatment, Suboxone itself can lead to dependency or misuse if not strictly used as prescribed. This can complicate recovery and create additional challenges for users.
If you've experienced Suboxone-related tooth decay or any other serious side effects, such as severe dental injuries, liver damage, or dependency, you may be entitled to financial compensation.
Filing a Suboxone lawsuit allows you to seek justice for your suffering and holds the manufacturer accountable for failing to adequately warn about these serious risks.
Don't wait to take action—contact LitigationConnect Suboxone lawyers online or at (833) 552-7274 for a free consultation.
How Do I Know If I Am Experiencing Side Effects From Suboxone Use?
If you've used Suboxone and are noticing unusual symptoms or severe dental decay, you might be suffering injuries related to the medication's serious side effects. Look out for signs such as:
- Tooth decay, cavities, gum disease, tooth loss, or other dental injuries that progress rapidly even with good oral hygiene.
- Symptoms like abdominal pain, jaundice, or unusual fatigue may point to liver inflammation or damage.
- Difficulty breathing, shallow breaths, or worsening respiratory symptoms, especially if combined with other sedatives.
It's not always easy to pinpoint the cause of your health problems. Suboxone injuries may develop over time, leaving you uncertain about what's to blame.
Contacting a mass tort attorney from the LitigationConnect network of legal professionals can help connect Suboxone users with experienced healthcare experts who can evaluate your condition and provide essential medical documentation to support your claim.
Why Are People Suing Over Suboxone-Related Dental Injuries?
Individuals are pursuing a Suboxone class action lawsuit due to significant injuries and the failure of its manufacturer, Indivior, to prioritize consumer safety. These lawsuits are anchored in key product liability principles, highlighting the following legal concerns:
- Failure to warn: Indivior is accused of failing to provide adequate warnings about the risks linked to Suboxone's sublingual delivery method. Under strict liability laws in states like California, manufacturers are obligated to disclose foreseeable risks to protect consumers, even if negligence isn't proven.
- Defective design: Plaintiffs argue that Suboxone's design is inherently flawed. Its sublingual administration exposes teeth and gums to prolonged chemical interactions, leading to accelerated decay. Alternatives like pills or transdermal patches may have significantly reduced this harm, showcasing that a safer design was feasible.
- Negligence and insufficient testing: Lawsuits claim that Indivior neglected its duty to conduct comprehensive, long-term safety studies on Suboxone's effects, particularly regarding dental health. Early patient complaints and dental injury reports, surfacing years before the FDA's 2022 warning, raise serious concerns about the company's failure to act and protect users.
- Preventable harm: Simple precautions, like advising users to rinse their mouths after taking Suboxone, could have mitigated dental damage. Plaintiffs emphasize that the lack of these measures highlights the preventable nature of these injuries.
- Deceptive marketing: Legal actions also allege that Indivior misled consumers by overstating Suboxone's safety and focusing on its effectiveness in treating opioid addiction while downplaying its severe risks, such as dependency and dental complications.
These allegations underscore the importance of holding manufacturers accountable for the harm caused by defective design, insufficient warnings, and negligence.
If you've experienced dental injuries or other adverse effects from Suboxone, pursuing a lawsuit could help achieve justice and compensation while prompting higher safety standards. Contact LitigationConnect today for a free consultation to explore your legal options.
How Much Could You Recover in a Suboxone Lawsuit?
If you've suffered harm from Suboxone, pursuing compensation can help cover the wide range of damages caused by its harmful side effects. While every case is unique, plaintiffs in Suboxone lawsuits may be able to recover compensation across multiple categories:
- Medical costs: Covers treatments related to your injuries, such as dental procedures or care for conditions like liver damage. These expenses can add up quickly and may include current and future medical needs.
- Lost wages: Provides compensation for income you've missed out on due to recovery time or ongoing injury-related complications that impacted your ability to work.
- Diminished earning potential: Accounts for the long-term financial impact of injuries that make it harder to return to work or continue your previous career path.
- Pain and suffering: Reflects the emotional and physical toll of injuries like dental damage, severe pain, or ongoing health struggles. This category acknowledges the distress these side effects can cause in your daily life.
- Loss of quality of life: Compensates for how your injuries may have altered your ability to enjoy life, maintain relationships, or carry out everyday activities.
- Punitive damages: May be awarded in cases where the manufacturer's actions were found to be reckless, such as ignoring known risks or misleading consumers. These damages act as a financial penalty and a deterrent against similar future behavior.
The value of your case depends on factors like the severity of your injuries, the documented connection to Suboxone, and state-specific laws. Consulting with a skilled product liability attorney can help you fully understand the range of compensation you might be eligible for while ensuring no aspect of your claim goes overlooked.
Explore every avenue for financial recovery with the help of an experienced legal team. Contact LitigationConnect today at (833) 552-7274 or through our online form for a free consultation. Taking this step can put you closer to the compensation you need to rebuild your life.
Are You Eligible to Join the Suboxone Lawsuit?
Many consumers are unsure if their circumstances qualify, and it's not always obvious how legal standards apply to your situation. However, you should never assume you don't have a case before exploring your options with a professional who understands the nuances of mass tort claims.
Eligibility requirements often depend on key factors, such as:
- Documented Suboxone use: Proof that you were prescribed and used Suboxone, such as medical records, pharmacy receipts, or prescription history.
- Evidence of harm: Injuries like severe dental damage, liver complications, or respiratory issues linked to Suboxone use. Detailed medical documentation and photos can strengthen your claim.
- Lack of adequate warnings: Instances where you weren't informed of the risks associated with Suboxone, particularly its connection to dental injuries or other side effects.
- Timely filing: Filing your claim within the appropriate statute of limitations for your state, which typically ranges from 1 to 6 years.
- Impact on daily life: Injuries that have caused significant disruption to your quality of life, ability to work, or personal relationships.
Understanding these factors is the first step to determining whether your situation meets the criteria for joining a Suboxone lawsuit. However, every case is different, and speaking with a legal professional ensures that no potential avenue of compensation is overlooked.
Suboxone Lawsuit FAQs
Is it too late to file a claim if I took Suboxone years ago?
Statutes of limitations vary by state, usually from 1 to 6 years. Still, certain factors could impact when that clock starts ticking for your case. Speaking with an attorney could help clarify your options and ensure no deadlines are missed.
What if I didn't notice any side effects while using Suboxone?
Even without immediate symptoms, injuries like dental decay or liver problems can develop and worsen over time. If you're experiencing health issues now, it's worth exploring whether they could be linked to your past use of Suboxone. A lawyer can help assess whether your case has merit, even if the connection initially seems unclear to you.
How much evidence do I need to build a strong case?
While every case is unique, having documentation such as medical records, pharmacy receipts, or photos of your injuries can strengthen your claim. You don't need to have all the answers or evidence upfront—that's where an experienced legal team comes in to guide you through the process and help gather what's needed.
Could joining a lawsuit hurt my chances of recovering financially?
Mass tort litigation is designed to benefit individuals by streamlining cases with common issues, which can increase efficiency while protecting your unique claim. Joining a lawsuit doesn't risk your financial recovery; on the contrary, it could strengthen your position by aligning your case with others who have suffered similar harm.
What if I feel unsure about pursuing legal action?
Feeling uncertain is completely understandable, especially when facing large corporations or unfamiliar legal processes. But filing a claim isn't just about compensation; it's also about holding manufacturers accountable when they fail to prioritize consumer safety. Exploring your options with a knowledgeable attorney can help you understand your rights and decide what's best for your future.
LitigationConnect | Helping You Take on Suboxone Cases With Confidence
Suboxone cases may require extensive evidence and legal support to establish the link between your injuries and the drug's harmful effects. Navigating these challenges without skilled representation could jeopardize your chance to recover fair compensation.
LitigationConnect is dedicated to connecting you with experienced mass tort attorneys who understand the unique complexities of Suboxone lawsuits. With their guidance, you can focus on your recovery while they advocate for the justice you deserve.
Don't wait to take control of your future. Call LitigationConnect today at (833) 552-7274 or complete our online form for a free consultation.